TIME IS LIMITED TO FILE A CLAIM – ACT NOW
TIME MAY BE LIMITED TO FILE!
TIME MAY BE LIMITED TO FILE!
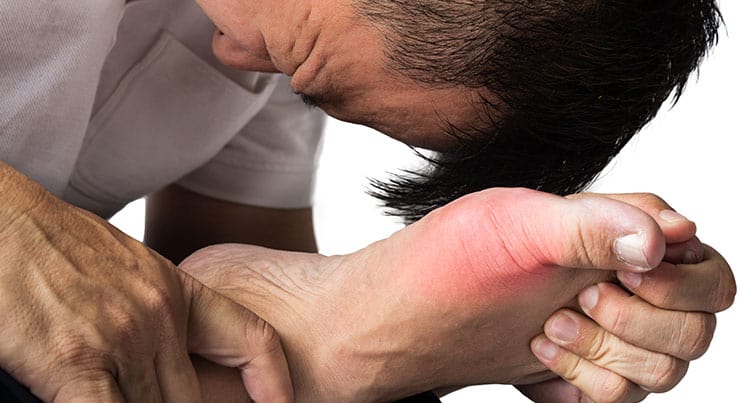
Did you or a loved one suffer complications after a Cartiva implant?
You may be entitled to significant financial compensation.
The Cartiva implant is a mainly water-based implant. It is placed in the great toe joint with a hole that is made into the metatarsal head. The implant is pressed fit into the hole and acts similar to regular cartilage, allowing motion of the joint.
Risks associated with implantation of hemiarthroplasty devices or Cartiva Synthetic Cartilage Implant include:
- infection
- inflammation, pain, swelling
- effusion
- joint irritation
- fibrosis
- joint instability, joint malalignment
- periarticular cyst
- bone cyst, bone loss
- sesamoid bone(s) irritation, sesamoid bone(s) fracture
- metatarsal bone fracture
- osteonecrosis
- avascular necrosis
- implant fracture, implant loosening, implant dislocation implant dislodgement, implant subsidence
- revision or conversion to fusion
- allergic reaction to polyvinyl alcohol (PVA)
- progressive osteoarthritis (OA)
- incorrect implant placement
- damage to adjacent or surrounding tissues
Get Your Free Case Evaluation Today
Sign up for a Confidential Case Review to find out if you’re eligible for financial compensation.
By clicking the “SEE IF I QUALIFY” button above, you agree that you will be contacted by telephone, email SMS/Text Message (Data Rates May Apply), even if you are on a federal or state Do Not Call registry, to confirm the accuracy of the information you submit and verify if you qualify.
Cartiva Implant background and development?

Cartiva, Inc. is a medical device company based in Alpharetta, Ga. Cartiva, Inc. gained FDA premarket approval for Cartiva SCI (surgical cartilage implant) in 2016 with specific indications for treatment of stage four hallux limitus, also known as hallux rigidus. Cartiva, Inc. sold their Cartiva SCI to Wright Medical Inc in a deal valued at $435 million which closed in the final quarter of 2018.
Cartiva, Inc. has provided the medical community with volumes of literature regarding Cartiva SCI through peer-reviewed journal articles and the MOTION study, a study of 197 patients at twelve centers. Take a few minutes to review the literature and I think you’ll be impressed. I was until I started using Cartiva® Implants.
WHAT IS CARTIVA?

The Cartiva implant is a mainly water-based implant. It is placed in the great toe joint with a hole that is made into the metatarsal head. The implant is pressed fit into the hole and acts similar to regular cartilage, allowing motion of the joint.
The procedure is extremely easy to perform and reproducible in the operating room. It is also easy to teach and patients have a fairly rapid recovery after the surgery.
The original studies performed to get FDA clearance was extensive, with over 10 surgeons involved in the study. The results were amazing in the study. Almost all of the patients had pain relief and would do the procedure over again. The study blew my colleagues and me away.
four reasons Cartiva® Implants are prone to impaction failure
Smooth implant surface
The Cartiva implant is made of a remarkably inert substance that shows very little reactivity in the body because the implant is very smooth on all surfaces it creates a functional flaw in the design.
Violation of the subchondral plate
Loss of the support of the subchondral plate with no alternative means of support (like the winged structure of the body of the Swanson Implant) places the Cartiva Implant into the soft metaphyseal bone and is prone to impaction failure.
Lack of osteointegration
Osteointegration, or ingrowth of bone into the implant is described by Rahyussalim as an essential aspect of implant stability. Osteointegration would seem to be an additional starting point for the redesign of the Cartiva Implant.
Placement in bone too soft for support
Over the lifetime of the implant (let’s say 20 years), what is the force applied to the implant and how supportive is the metaphyseal bone? The supporting bone is too weak to provide meaningful, long-term support to resist the load applied to the implant.
We Can Help
Our network of attorneys have been helping individuals, like you, for years.
Do I Have A Case
If you or a loved one have suffered from adverse effects from a Cartiva Implant, you may be entitled to financial compensation.
Experienced Attorneys
Our team of experts has extensive experience fighting to hold Medical manufacturers and distributers accountable.
Act Now
Contact us right away to set up a free, confidential and no-obligation case review. We’re ready to fight for you.
ATTORNEY ADVERTISEMENT
This website or its third-party tools use cookies, which are necessary for its functioning and required to achieve the purposes illustrated in the cookie policy, including the personalization and analysis of ads and content. If you want to learn more please refer to the cookie policy and privacy policy. You accept the use of cookies by scrolling this page, by clicking a link or button or by continuing to browse otherwise.
27475 Ynez Rd #708, Temecula, CA 92591
